Translate this page into:
High-definition transcranial direct current stimulation for treating cognitive and negative symptoms in chronic schizophrenia – A sham-controlled proof of concept study

*Corresponding author: Bhawna Yadav, Department of Psychiatry, Central Institute of Psychiatry, Ranchi, Jharkhand, India. bhawnayadav1712@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Yadav B, Goyal N, Roy C, Ramamoorthy D. High-definition transcranial direct current stimulation for treating cognitive and negative symptoms in chronic schizophrenia – A sham-controlled proof of concept study. Arch Biol Psychiatry 2023;1:46-53. doi: 10.25259/ABP_20_2023
Abstract
Objectives:
Cognitive and negative symptoms are core symptoms of schizophrenia affecting interpersonal and socio-occupational functioning. Impaired dorsolateral prefrontal cortex (DLPFC) function is implicated in negative and cognitive symptoms. Conventional transcranial direct current stimulation (tDCS) to DLPFC has attracted interest as an add-on treatment for these symptoms. High-definition tDCS (HD-tDCS), an optimized form of tDCS, has the potential for more focalized neuromodulation. Studies suggest that an increased number of sessions may increase the effectiveness of stimulation. Hence, we aimed to evaluate the efficacy of 20 sessions of HD-tDCS over the left DLPFC in the improvement of cognitive and negative symptoms in chronic schizophrenia (>2 years continuous illness).
Material and Methods:
Twenty patients with chronic schizophrenia with predominantly cognitive and negative symptoms were enrolled in this sham-controlled trial. Participants received 20 sessions of HD-tDCS at 2 mA for 20 min, that is, twice daily over 10 days. Montreal cognitive assessment and scale for assessment of negative symptoms were used to assess outcome variables. Assessments were carried out at baseline, 2 weeks, and 6 weeks, respectively.
Results:
Significant improvement was noted in both active and sham groups across all outcome variables over time. However, a statistically significant decrease in negative symptoms in the active group was noted, which was maintained at the end of 6 weeks, but there was no statistically significant improvement in cognitive symptoms between the active and sham groups at 6 weeks. The stimulation protocol was well tolerated.
Conclusion:
HD-tDCS has substantial potential in the treatment of negative symptoms; however, its role in cognitive symptoms needs further evaluation.
Keywords
Cognitive symptoms
Negative symptoms
Schizophrenia
HD-tDCS
INTRODUCTION
Schizophrenia, a highly debilitating mental disorder, impacts approximately 1% of the population.[1] This condition is marked by various cognitive factors that contribute to widespread cognitive impairment in individuals diagnosed with schizophrenia. These factors encompass processing speed, attention and vigilance, working memory, verbal learning and memory, visual learning, memory, reasoning and problem-solving, as well as verbal and social cognition.[2] Cognitive impairments in Schizophrenia greatly debilitate and impair socio-occupational functioning.[3] While resistant to conventional treatments, the negative symptoms of schizophrenia – social withdrawal, decreased affective response, reduced interest, impaired social drive, and diminished sense of purpose – are enduring and more predictive of outcomes than positive symptoms.[4-6]
Prefrontal cortex (PFC) abnormalities are thought to affect cognitive and negative symptoms in patients with schizophrenia. Patients consistently show decreased dorsolateral PFC (DLPFC) activity during working memory tasks, where activation levels correlated to performance in many studies.[7,8] Patients with negative symptoms of schizophrenia also show lower metabolism in the DLPFC.[9] Therefore, it is reasonable to assume that changes in PFC activity may play a role in the cognitive and negative symptoms of schizophrenia. Hypometabolism and hypoperfusion of the PFC have been seen in neuroimaging studies in patients with negative symptoms of schizophrenia suggesting hypofrontality, which is more pronounced on the left side.[10-12]
Neuromodulation techniques that are non-invasive, such as transcranial direct current stimulation (tDCS) and repetitive transcranial magnetic stimulation (rTMS), are being tried for the treatment of various psychiatric disorders. tDCS is a novel technique in which two electrodes are used to deliver a weak electrical current to the scalp. The anode leads to neuronal depolarization, and the cathode facilitates neuronal hyper-polarization.[13] tDCS-induced changes depend on the polarity of stimulation – anodal stimulation conventionally enhances excitability, while cathodal tDCS reduces it.[14,15] Conventional tDCS affects cortical activity in a relatively larger area than that covered by the target electrode as found in neuroimaging studies.[16]
As compared to other non-invasive neuromodulation techniques, tDCS is easy to use, cheap, and portable.[17] High-definition tDCS (HD-tDCS) has the possibility for precise and focal neuromodulation with better predictability of effects and is a betterment of conventional tDCS. Instead of saline-soaked sponges, HD-tDCS uses a network of small electrodes (1 cm) arranged in a 4 * 1 ring electrode configuration, leading to focalized stimulation according to the finite element model analysis based on high-resolution magnetic resonance imaging (MRI).[18] Conventional tDCS leads to the flow of current in wider brain areas, where the current might be spread diffusely and the greatest density of stimulation might not occur directly under the electrodes. HD-tDCS, on the other hand, allows an effective density of current mainly in the target area, leading to more focal, stronger, and longer-lasting effects than conventional tDCS.[19,20]
A meta-analysis of 31 studies found a moderate effect on negative symptoms of schizophrenia using either tDCS or rTMS of the frontal cortex.[21] A most recent meta-analysis done to assess the effects of tDCS on negative and cognitive symptoms in schizophrenia patients failed to show statistically significant results for cognitive symptoms; however, tDCS instead of HDt-DCS was used in the studies. Overall, the meta-analysis did not show a significant difference in improving negative symptoms between active and sham tDCS in patients with schizophrenia. However, in the analysis of studies with a greater frequency of stimulation, that is, twice daily, a significant difference was found in the effects of active stimulation as compared to sham tDCS suggesting a role for increasing the number of stimulations to improve effectiveness.[22]
A recent study on the effectiveness of adjunctive HD-tDCS in schizophrenia patients with negative symptoms stated that the initial reduction in negative symptoms was maintained over a 6-week follow-up and suggested an increase in the number of stimulations for further studies.[23] However, so far we could not find any published study on the use of 20 sessions of HDtDCS in the treatment of cognitive and negative symptoms of chronic schizophrenia (>2 years of continuous illness).[24] Our study aimed to evaluate the effectiveness of HD-tDCS to left DLPFC in treating cognitive and negative symptoms in patients with chronic schizophrenia.
MATERIAL AND METHODS
Participants
The study was conducted at the Brain Stimulation Lab, Center for Cognitive Neurosciences, Central Institute of Psychiatry, Ranchi, India. Subjects between the ages of 18 and 60 years, who met International Classification of Diseases, Tenth Edition (ICD-10) DCR criteria for schizophrenia, illness duration >2 years with continuous course, and right-handed, with a scale for assessment of negative symptoms (SANS) score of more than 20 were included in the study.[23] The handedness preference schedule was used to assess handedness.[25] The diagnosis of schizophrenia was established using ICD-10 after detailed clinical evaluation through clinical interviews.
The study excluded patients with substance dependence except for nicotine and caffeine, comorbid medical/ neurological disorders, and developmental delay, as well as those who were receiving depot antipsychotics to minimize confounding factors that could affect the study outcomes. Patients having metallic implants/parts and who had received electroconvulsive therapy in the past 6 months were also excluded from the study.
Before conducting the study, a thorough explanation of the research was provided to the participants, and written informed consent was taken following the approval of the protocol by the Institute’s Ethics Committee.
A total of 26 inpatients were initially assessed for eligibility. However, four patients did not cooperate in the beginning and were excluded from further participation. An additional two patients dropped out due to an increase in positive symptoms.
Ultimately, a group of 20 patients successfully completed the entire duration of the study, as illustrated in [Figure 1]. Throughout the study duration, the patients remained on stable doses of antipsychotic medication, as determined by the treating team.
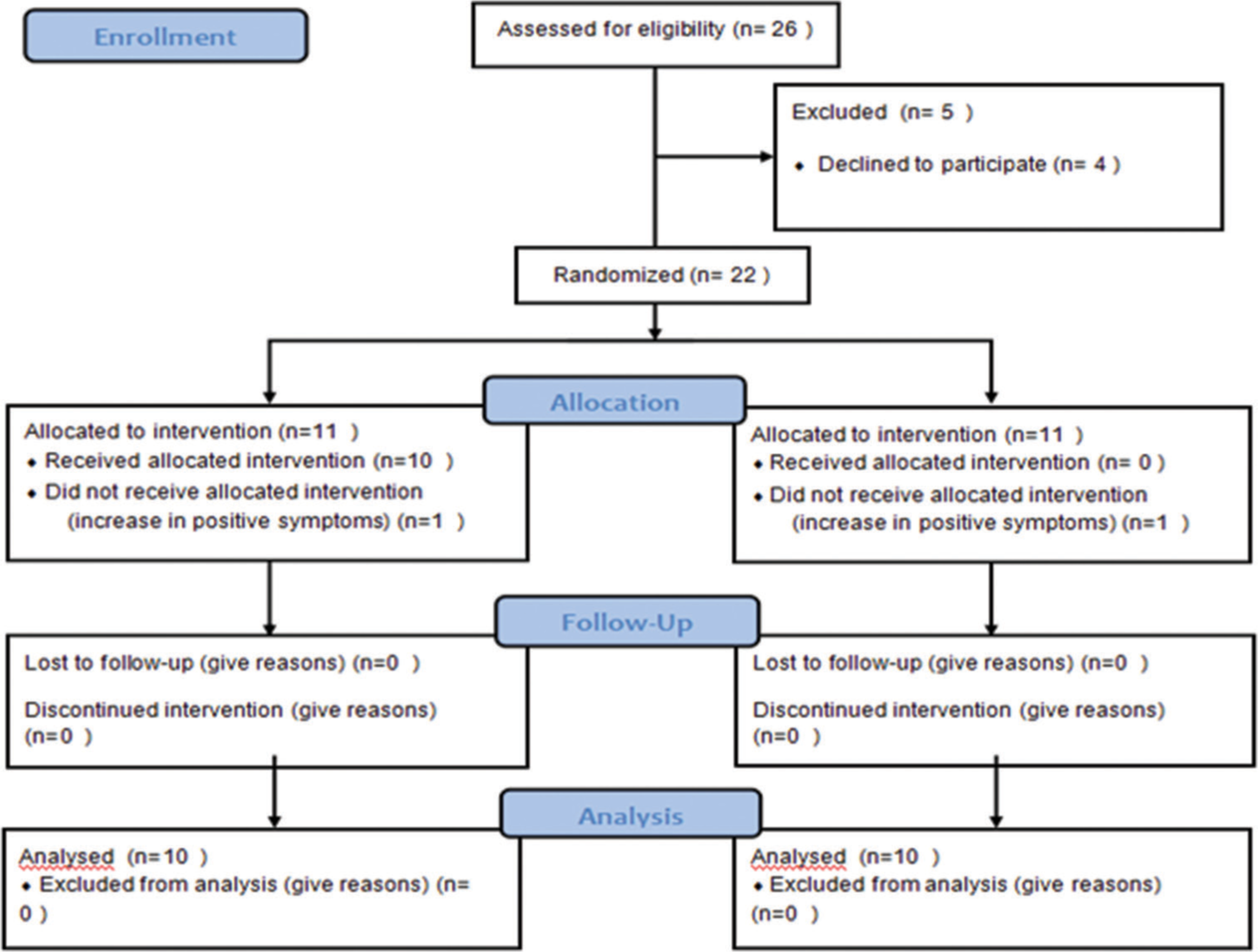
- CONSORT 2010 flow diagram.
Clinical assessment
The study utilized the Montreal Cognitive Assessment Scale [26] (MoCA) to assess cognitive symptom improvement, the SANS[27] to measure the severity of negative symptoms, and the clinical Global Impression-severity Scale [28] (CGI-S) for evaluating overall psychopathology. Depression screening was conducted using the Calgary Depression Scale for schizophrenia[29] with a cutoff score of <7.[23] Clinical ratings were performed at baseline before HDt-DCS sessions (day 1) and after 2 weeks and 6 weeks (4 weeks post-HD-tDCS) to evaluate the effects of HD-tDCS.
Design/intervention
This double-blind and sham-controlled study randomly allocated participants into two groups. Both evaluators and patients were unaware of the assignment of treatment to either the active or sham group. The active HD-tDCS stimulation was delivered using a wearable device called Starstim 32 from Neuroelectrics BE, Spain. The device wirelessly transmitted 32-channel data through Wi-Fi. NG Pistim electrodes that had a 1 cm radius were used in a 4 × 1 ring montage. The electrode placement on the scalp was facilitated by an electroencephalogram (EEG) cap and plastic casing for stability. The anode electrode (central electrode) was positioned at F3 (Left DLPFC) following the 10/10 montage of EEG electrode placement. Four return electrodes (FC1, F7, FC5, AF3) surrounded the central electrode, defining the modulation area [Figure 2]. Even though very low electric current can have local effects, studies have found that induced electric fields are grossly constrained to the cortical area circumscribed by the ring. [30] Throughout the sessions, side effects were monitored using a checklist specifically designed for HD-tDCS (HD-tDCS side effect checklist).[31]

- Simulated electric field maps produced over the left dorsolateral prefrontal cortex by a high-definition – transcranial direct current stimulation.
In line with previous research, the active group received stimulation of 2 mA with two sessions per day for 10 days. The stimulation included a 3-s ramp up and ramp down.[22,23] The full duration of each stimulation session was 20 min.[32,33] A minimum interval of 3 h separated two consecutive sessions. For the sham stimulation, electrodes were positioned in the same locations as in the active group. However, a 1 mA direct current was delivered for 30 s with a 3-s ramp up and ramp down to give a sensation of stimulation while reducing actual stimulatory effects.[34] Enveloped randomization was used for allocating participants to active and sham groups.
The study received ethical approval from the Institute Ethics Committee of the Central Institute of Psychiatry, Ranchi. The trial protocol was registered with the Clinical Trials Registry – India (CTRI) at http://ctri.nic.in/Clinicaltrials/login.php with the number CTRI/2021/07/034759.
Statistical analysis
The data analysis was conducted with the Statistical Package for the Social Sciences-version 25.0 (SPSS-25.0), for Windows®. Descriptive statistics were used to compare the demographic and clinical characteristics of the sample. Independent t-tests and Chi-square tests were utilized, as appropriate, to examine group differences in sample characteristics. To assess the overall treatment effect over time for both groups, a generalized linear model-repeated measures analysis was employed, with treatment as the between-group factor and time as the within-subject factor. The Greenhouse–Geisser test was applied to correct for sphericity. A significance level (P < 0.05) (two-tailed) was considered statistically significant.
RESULTS
Out of the 20 patients who completed the study, ten received active HD-tDCS stimulation, while the other ten received sham stimulation. The allocation of participants to the active and sham groups was determined using the sealed envelope method. Sociodemographic and clinical characteristics were compared between the active and sham groups, and the analysis showed no significant differences in terms of age, duration of illness, income, education, sex, religion, marital status, or choice of drug [Tables 1 and 2].
| Variables | Active n=10 Mean±SD | Sham n=10 Mean±SD | t | df | P |
|---|---|---|---|---|---|
| Age (in years) | 29.80±7.34 | 32.50±6.94 | −0.84 | 18 | 0.41 |
| Years of education | 13.20±2.44 | 13.20±2.14 | 0.00 | 18 | 1 |
| Duration of illness (in years) | 7.32±5.22 | 10.80±3.99 | −1.39 | 18 | 0.18 |
| Income (in rupees) | 22000±12463.27 | 23200±15604.84 | −0.190 | 18 | 0.85 |
P=NS. SD: Standard deviation
| Variables | Active (n=10) (%) | Sham (n=10) (%) |
t | df | P |
|---|---|---|---|---|---|
| Sex | |||||
| Male | 8 (80) | 8 (80) | 0.00 | 1 | 1 |
| Female | 2 (20) | 2 (20) | |||
| Religion | |||||
| Hindu | 9 (90) | 8 (80) | 3.05 | 2 | 2.17 |
| Muslim | 0 (0) | 2 (20) | |||
| Christian | 1 (10) | 0 (0) | |||
| Marital status | |||||
| Unmarried | 4 (40) | 3 (30) | 1.58 | 3 | 0.66 |
| Married | 5 (50) | 4 (40) | |||
| Divorced | 0 (0) | 1 (10) | |||
| Widowed | 1 (10) | 2 (20) | |||
| Drug | |||||
| Olanzapine | 1 (10) | 2 (20) | 3.33 | 3 | 0.34 |
| Risperidone | 3 (30) | 1 (10) | |||
| Clozapine | 3 (30) | 6 (60) | |||
| Other | 3 (30) | 1 (10) |
P=NS
The safety and tolerability of HD-tDCS were assessed using a checklist developed by Eryilmaz et al.[31] Among the patients receiving active stimulation, itching at the site of stimulation was complained of by four patients during seven sessions total, while a burning sensation at the stimulation site was reported by two patients, each during a single session. No other major or debilitating side effects were reported. All reported side effects were transient and did not need discontinuation of sessions.
[Table 3] shows changes in psychopathology as seen by improvements in symptoms measured by changes in MoCA, SANS, and CGI-S scores over time. There was a significant improvement in cognitive symptoms as assessed using MoCA (F = 22.63; P < 0.001) with a good effect size (Partial eta squared = 0.72). A significant improvement was also noticed in the negative symptoms measured by SANS score (F = 15.39; P < 0.001) over time at 2 weeks in both active and sham groups, which was maintained even after 4 weeks of completion of HD-tDCS with a large effect size (Partial Eta squared = 0.64) and global severity as assessed using CGI-S (F = 49.50; P < 0.001) over time at 2 weeks in both active and sham groups with good effect sizes (Partial Eta Squared = 0.85) and the improvement was maintained at 4 weeks post HD-tDCS.
| Variable | Time | Active (n=10) Mean±SD | Sham (n=10) Mean±SD | Pillai’s trace F | P | Partial eta squared | Post hoc |
|---|---|---|---|---|---|---|---|
| SANS | Baseline1 | 57.20±18.81 | 57.40±19.65 | 15.39 | <0.001 | 0.64 | 1>2* |
| 2 weeks2 | 39.70±14.84 | 47.00±20.30 | 1>3* | ||||
| 6 weeks3 | 37.10±15.96 | 44.50±19.98 | 2>3* | ||||
| CGI-S | Baseline1 | 5.20±0.42 | 5.50±0.84 | 49.50 | <0.001 | 0.85 | 1>2* |
| 2 weeks2 | 3.90±0.73 | 4.40±0.96 | 1>3* | ||||
| 6 weeks3 | 3.60±1.07 | 4.10±0.99 | 2>3* | ||||
| MoCA | Baseline1 | 23.30±2.21 | 22.70±2.98 | 22.63 | <0.001 | 0.72 | 1>2* |
| 2 weeks2 | 24.80±1.81 | 24.30±2.11 | 1>3* | ||||
| 6 weeks3 | 24.70±7.94 | 24.20±2.04 | 2>3* |
We failed to find any improvement in cognitive symptoms between active and sham HD-tDCS groups as seen on MoCA (F = 0.025; P = 0.905) [Figure 3a]. However, Group*Outcome Interaction as mentioned in [Table 4] revealed that active HD-tDCS was better at reducing negative symptoms as assessed by SANS (F = 1.73; P = 0.02) with a large effect size (Partial Eta Squared = 0.19) [Figure 3b] and clinical global improvement as assessed by CGI-S (F = 0.27; P = 0.05) with a large effect size (Partial Eta Squared = 0.20) [Figure 3c]. This implies that 20 sessions of HD-tDCS led to significant improvement in negative symptoms and global change in severity, although we failed to establish that active HD-tDCS was better than sham HD-tDCS for cognitive symptoms of schizophrenia.
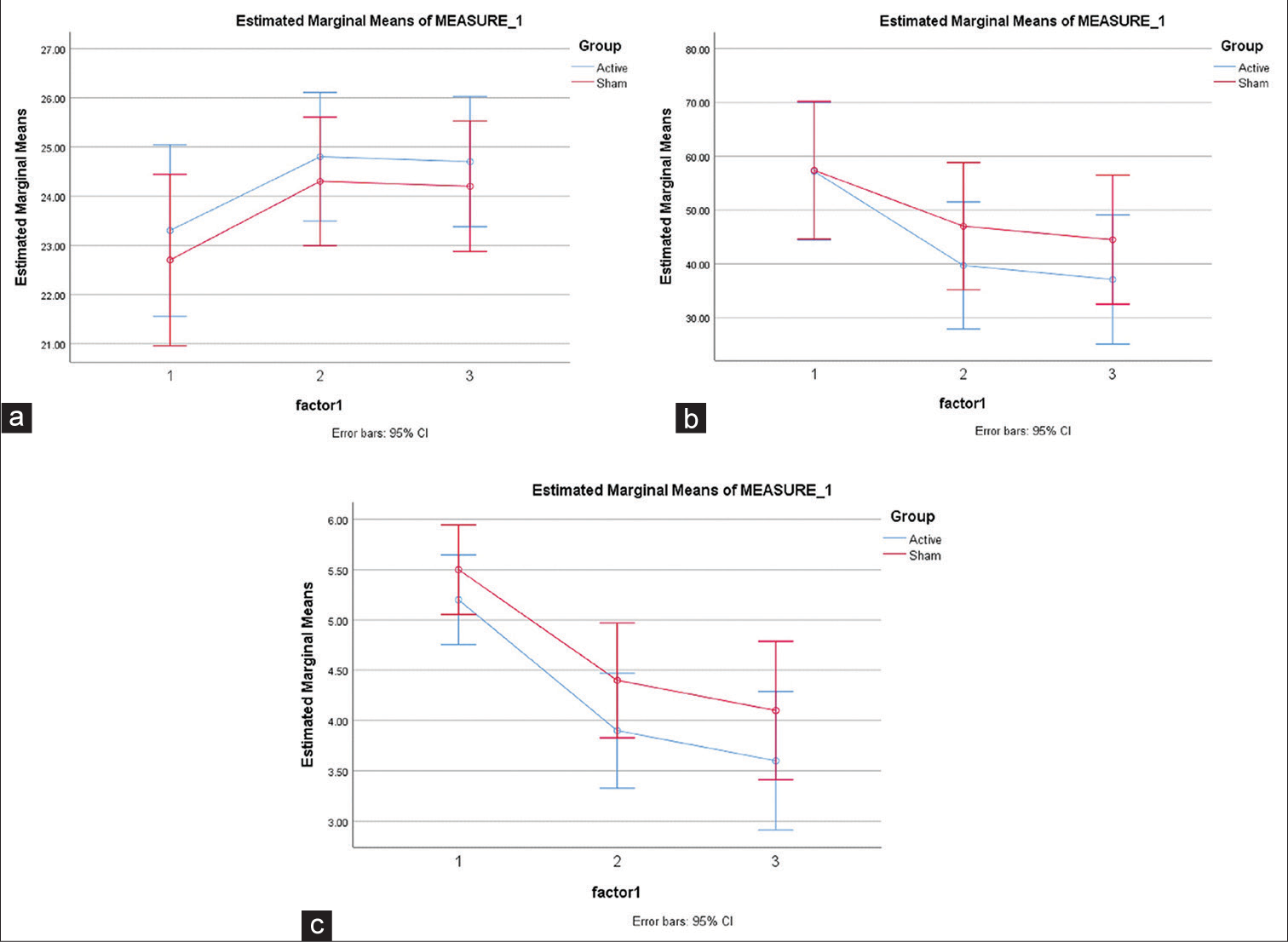
- (a) Estimated marginal means of Montreal cognitive assessment scale, (b) estimated marginal means of scale for assessment of negative symptoms, and (c) estimated marginal means of clinical global impression severity.
| Group*outcome | G.G.F. | df | P | Partial eta squared |
|---|---|---|---|---|
| Group*SANS | 1.73 | 2 | 0.02 | 0.19 |
| Group*MoCA | 0.025 | 2 | 0.905 | 0.001 |
| Group*CGI-S | 0.277 | 2 | 0.05 | 0.205 |
DISCUSSION
Significant advancements have been made in the treatment of schizophrenia in recent years. However, addressing negative symptoms and cognitive deficits remains a significant challenge in patient care. The treatment of cognitive symptoms has received limited attention, highlighting the need for effective interventions.[35] Existing therapies for negative symptoms in schizophrenia lack robust efficacy. Conversely, research on improving cognitive symptoms is limited, primarily relying on anecdotal evidence. This highlights the importance of further investigation and the development of effective treatments in this area.
Non-invasive brain stimulation techniques like tDCS offer promise as alternative treatments for medication-resistant symptoms in schizophrenia, particularly negative and cognitive symptoms. However, the optimal implementation of these treatments is still being explored. The previous studies utilizing conventional tDCS have demonstrated notable positive effects, although there have also been instances of negative outcomes with similar or slightly varied approaches.
We aimed to investigate the potential of HD-tDCS as a novel method for enhancing cognitive and negative symptoms in schizophrenia. Our study is the first to utilize more than ten sessions with a ring electrode arrangement to target symptom improvement in this population. The previous studies have primarily used conventional tDCS or HD-tDCS with limited session duration.[36,37] In contrast, we assessed the effects of 20 sessions of HD-tDCS specifically targeting the left DLPFC in chronic schizophrenia.
One advantage of our study was the inclusion of a sham control group, reducing unsystematic factors that could influence treatment outcomes. While previous studies have explored the utility of HD-tDCS for managing negative symptoms, none have utilized 20 sessions of HD-tDCS. Results from previous studies have been inconsistent, with some demonstrating significant improvement in negative symptoms while others failed to replicate these findings.[38-40]
Our study found significant improvements in cognitive, negative, and overall symptom severity over time in both the active and sham groups. These effects could be attributed to medication, the HD-tDCS intervention, or even a placebo response in both groups. General linear model-repeated measures ANOVA revealed significant differences in the changes in SANS and CGI-S scores over time between the active and sham groups. These findings are consistent with another study conducted by Dharani and colleagues, which also showed significant reductions in SANS and PANSS scores between the active and sham HD-tDCS groups, persisting at the 6-week follow-up.[23]
Top of form
Contrary to our initial expectations, we were unsuccessful in finding any therapeutic effects of 20 sessions of HDtDCS stimulation of the left DLPFC on cognitive symptoms of chronic schizophrenia. Gomes et al.[35] investigated the therapeutic potential of tDCS with anodal stimulation to the left DLPFC and cathodal stimulation to the right DLPFC and found no improvement in cognitive performance after active versus sham tDCS. HD-tDCS, even though well tolerated in this clinical population, failed to produce any therapeutic benefits.
There may be several reasons why we have failed to demonstrate therapeutic effects on cognitive symptoms in chronic schizophrenia. The first was a small sample size, which may be a potential limitation of our study. A recently done meta-analysis showed significant improvement in negative symptoms and a trend toward significance in improvement in cognitive symptoms with tDCS using twice daily stimulation.[22] Perhaps, longer treatment protocols with larger sample sizes might show the beneficial effects of HD-tDCS.
Another limitation of our study was that the person administering the current was aware of the active and sham participants, as active and sham stimulation protocols had to be selected manually in the system. However, both the patient and the rater were unaware of the same.
The therapeutic potential of HD-tDCS in schizophrenia is rooted in its capacity to induce cortical plasticity. Through subthreshold polarization of neuronal membranes using weak direct currents, tDCS can non-invasively promote plastic changes in the brain. The direction of these changes depends on the stimulation polarity, with anodal stimulation typically enhancing excitability and cathodal stimulation reduces it.[14] A comparative study of conventional tDCS and HD-tDCS demonstrated that both methods induced excitability enhancement following anodal stimulation. However, HDtDCS resulted in delayed peak plastic changes at 30 min and long-lasting post-stimulation effects exceeding 2 h.[30]
Given previous research indicating greater left-sided hypofrontality in schizophrenia, it was hypothesized that anodal HD-tDCS targeting the left DLPFC could enhance excitability and induce neuroplasticity, potentially leading to improvements in negative symptoms. This hypothesis aligns with findings from previous studies that have reported similar positive effects.[41,42]
Our study using 20 sessions of HD-tDCS with two sessions daily for 10 days with a 6-week follow-up assessing cognitive and negative symptoms in schizophrenia might be a way forward for future studies using a larger sample size, a higher current dosage, and more controlled conditions, along with the use of tools that allow for a more detailed evaluation of cognitive functions. Future research should explore the neurophysiobiological mechanisms of HD-tDCS to develop personalized stimulation protocols for improved efficacy in schizophrenia treatment.
CONCLUSION
Our study adds to the evidence of the effectiveness of adjuvant HD-tDCS in reducing negative symptoms of schizophrenia; however, we failed to find a reduction of cognitive symptoms in patients using a novel protocol implying the use of HDtDCS which appeared to be safe. Given the inconsistent findings in the existing literature, further exploration is necessary to determine the ideal combination of HD-tDCS parameters for effectively treating cognitive symptoms in schizophrenia. This exploration should involve larger sample sizes and utilize objective markers, such as task-based functional MRI and similar techniques, to measure improvements in a more objective and reliable manner.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Financial support and sponsorship
Nil.
References
- The epidemiology of schizophrenia: Replacing dogma with knowledge. Dialogues Clin Neurosci. 2010;12:305-15.
- [CrossRef] [PubMed] [Google Scholar]
- Identification of separable cognitive factors in schizophrenia. Schizophr Res. 2004;72:29-39.
- [CrossRef] [PubMed] [Google Scholar]
- Cognitive impairment in schizophrenia is the core of the disorder. Crit Rev Neurobiol. 2000;14:1-21.
- [CrossRef] [PubMed] [Google Scholar]
- Persistent negative symptoms in schizophrenia: An overview. Schizophr Bull. 2007;33:1013-22.
- [CrossRef] [PubMed] [Google Scholar]
- Duration of untreated psychosis and negative symptoms-a systematic review and meta-analysis of individual patient data. Schizophr Res. 2012;142:12-9.
- [CrossRef] [PubMed] [Google Scholar]
- Self-efficacy and functional status in schizophrenia: Relationship to insight, cognition and negative symptoms. Schizophr Res. 2013;145:69-74.
- [CrossRef] [PubMed] [Google Scholar]
- Physiologic dysfunction of dorsolateral prefrontal cortex in schizophrenia. I. Regional cerebral blood flow evidence. Arch Gen Psychiatry. 1986;43:114-24.
- [CrossRef] [PubMed] [Google Scholar]
- Complexity of prefrontal cortical dysfunction in schizophrenia: More than up or down. Am J Psychiatry. 2003;160:2209-15.
- [CrossRef] [PubMed] [Google Scholar]
- Negative symptoms and hypofrontality in chronic schizophrenia. Arch Gen Psychiatry. 1992;49:959-65.
- [CrossRef] [PubMed] [Google Scholar]
- Left hypofrontality correlates with blunted affect in schizophrenia. Jpn J Psychiatry Neurol. 1992;46:653-7.
- [CrossRef] [PubMed] [Google Scholar]
- Cerebral perfusion correlates of negative symptomatology and Parkinsonism in a sample of treatment-refractory schizophrenics: An exploratory 99mTcHMPAO SPET study. Schizophr Res. 1997;25:11-20.
- [CrossRef] [PubMed] [Google Scholar]
- Regional cerebral blood flow SPECT study, at rest and during Wisconsin Card Sorting Test (WCST) performance, in schizophrenia naive patients or treated with atypical neuroleptics. Actas Esp Psiquiatr. 2005;33:343-51.
- [Google Scholar]
- Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol. 2000;527:633-9.
- [CrossRef] [PubMed] [Google Scholar]
- Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology. 2001;57:1899-901.
- [CrossRef] [PubMed] [Google Scholar]
- Level of action of cathodal DC polarisation induced inhibition of the human motor cortex. Clin Neurophysiol. 2003;114:600-4.
- [CrossRef] [PubMed] [Google Scholar]
- Transcranial direct current stimulation of the unaffected hemisphere in stroke patients. Neuroreport. 2005;16:1551-5.
- [CrossRef] [PubMed] [Google Scholar]
- Transcranial direct current stimulation: State of the art 2008. Brain Stimulat. 2008;1:206-23.
- [CrossRef] [PubMed] [Google Scholar]
- Gyri-precise head model of transcranial direct current stimulation: Improved spatial focality using a ring electrode versus conventional rectangular pad. Brain Stimul. 2009;2:201-7.
- [CrossRef] [PubMed] [Google Scholar]
- Physiological and modeling evidence for focal transcranial electrical brain stimulation in humans: A basis for high-definition tDCS. Neuroimage. 2013;74:266-75.
- [CrossRef] [PubMed] [Google Scholar]
- A finite element analysis of the effect of electrode area and inter-electrode distance on the spatial distribution of the current density in tDCS. J Neural Eng. 2011;8:66017.
- [CrossRef] [PubMed] [Google Scholar]
- Non-invasive brain stimulation for negative symptoms in schizophrenia: An updated systematic review and meta-analysis. Schizophr Res. 2018;197:34-44.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy of transcranial direct current stimulation in ameliorating negative symptoms and cognitive impairments in schizophrenia: A systematic review and meta-analysis. Schizophr Res. 2020;224:2-10.
- [CrossRef] [PubMed] [Google Scholar]
- Adjuvant high-definition transcranial direct current stimulation for negative symptoms in schizophrenia: A pilot study. J ECT. 2021;37:195-201.
- [CrossRef] [PubMed] [Google Scholar]
- Lateral cerebral ventricular enlargement in chronic schizophrenia. Arch Gen Psychiatry. 1979;36:735-9.
- [CrossRef] [PubMed] [Google Scholar]
- The Montreal cognitive assessment, MoCA: A brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695-9.
- [CrossRef] [PubMed] [Google Scholar]
- Negative symptoms in schizophrenia: Definition and reliability. Arch Gen Psychiatry. 1982;39:784-8.
- [CrossRef] [PubMed] [Google Scholar]
- The clinical global impression severity and improvement scales In: ECDEU Assessment Manual for Psychopharmacology. Rockville, MD: US Department of Health, Education and Welfare Publication (ADM); 1976. p. :76-338.
- [CrossRef] [Google Scholar]
- A depression rating scale for schizophrenics. Schizophr Res. 1990;3:247-51.
- [CrossRef] [PubMed] [Google Scholar]
- Comparing cortical plasticity induced by conventional and high-definition 4×1 ring tDCS: A neurophysiological study. Brain Stimul. 2013;6:644-8.
- [CrossRef] [PubMed] [Google Scholar]
- Adverse effects of transcranial direct current stimulation (TDCS) in a group of psychiatric patients. Sch J Appl Med Sci. 2014;2:294-7.
- [Google Scholar]
- A randomized, double-blind clinical trial on the efficacy of cortical direct current stimulation for the treatment of major depression. Int J Neuropsychopharmacol. 2008;11:249-54.
- [CrossRef] [PubMed] [Google Scholar]
- Transcranial DC stimulation (tDCS): A tool for double-blind sham-controlled clinical studies in brain stimulation. Clin Neurophysiol. 2006;117:845-50.
- [CrossRef] [PubMed] [Google Scholar]
- Cognitive deficit in schizophrenia: From etiology to novel treatments. Int J Mol Sci. 2021;22:9905.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of transcranial direct current stimulation on working memory and negative symptoms in schizophrenia: A phase II randomized sham-controlled trial. Schizophr Res Cogn. 2018;12:20-8.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy and safety of transcranial direct current stimulation for treating negative symptoms in schizophrenia: A randomized clinical trial. JAMA Psychiatry. 2020;77:121-9.
- [CrossRef] [PubMed] [Google Scholar]
- Examining transcranial direct-current stimulation (tDCS) as a treatment for hallucinations in schizophrenia. Am J Psychiatry. 2012;169:719-24.
- [CrossRef] [Google Scholar]
- Prefrontal transcranial direct current stimulation for treatment of schizophrenia with predominant negative symptoms: A double-blind, sham controlled proof-of-concept study. Schizophr Bull. 2016;42:1253-61.
- [CrossRef] [PubMed] [Google Scholar]
- A negative pilot study of daily bimodal transcranial direct current stimulation in schizophrenia. Brain Stimul. 2014;7:813-6.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of transcranial direct current stimulation (tDCS) on cognition, symptoms, and smoking in schizophrenia: A randomized controlled study. Schizophr Res. 2015;168:260-6.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical research with transcranial direct current stimulation (tDCS): Challenges and future directions. Brain Stimul. 2012;5:175-95.
- [CrossRef] [PubMed] [Google Scholar]
- Modulation of brain activity with noninvasive transcranial direct current stimulation (tDCS): Clinical applications and safety concerns. Front Psychol. 2017;8:685.
- [CrossRef] [PubMed] [Google Scholar]








