Translate this page into:
Efficacy of adjunctive theta burst transcranial magnetic stimulation in acute mania: A randomized and placebo-controlled study

*Corresponding author: Shobit Garg, Department of Psychiatry, Shri Guru Ram Rai Institute of Medical and Health Sciences, Dehradun, Uttarakhand, India. shobit.garg@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Bhatia A, Garg S, Tyagi P, Pandey E. Efficacy of adjunctive theta burst transcranial magnetic stimulation in acute mania: A randomized and placebo-controlled study. Arch Biol Psychiatry 2023;1:11-8.
Abstract
Objectives:
Transcranial magnetic stimulation (TMS) has been suggested as a non-invasive stimulation treatment modality in bipolar disorder. However, the efficacy of repetitiver TMS in acute phase mania is unclear. The aim of this study is to assess the efficacy of theta burst stimulation (TBS) as an add-on therapy in the treatment of acute phase mania stimulation both right and left dorsolateral prefrontal cortex (DLPFC) in a randomized and sham-controlled design.
Material and Methods:
Forty right-handed patients between 18 and 59 years were randomly allocated to active and sham groups. Then, intermittent TBS (right DLPFC) and cTBS (left DLPFC) sessions (half an hour apart) were delivered in two sessions per day for 5 days in a week. Youngs Mania Rating Scale (YMRS) and Clinical global impression-Bipolar Disorder (CGI-BP) were assessed at baseline and at 7th and 21st days.
Results:
Our study fails to show a significant effect on of active stimulation in comparison to sham over psychopathology YMRS (F = 0.300; P ≤ 0.612), CGIBP-S (F = 0.432; P = 0.562), CGIBP-P (F = 0.202; P = 0.701), and CGIBP-W (F = 200; P = 0.705) in intention to treat protocol across 21 days. Minimal side effects were reported and none of the patients discontinued TBS citing side effects.
Conclusion:
We conclude that it is safe and well tolerated yet has inconclusive short-term therapeutic benefits. Trials using better localization technique with large sample, longer duration, and better dosing protocols are needed.
Keywords
Transcranial magnetic stimulation
Acute phase management
Bipolar disorder
Sham-controlled study
INTRODUCTION
Bipolar disorder is characterized by episodes of irritable or elevated mood (hypomanic or manic episodes) and periods of lack of energy and low mood (depressive episodes).[1] An essential component of a manic episode is that there is an “abnormally irritable, elevated, or expansive mood, and increased energy or activity lasting at least 1 week (or any duration if hospitalization is necessary), along with at least three symptoms from Criterion B (grandiosity, short sleep, pressure of speech, flight of ideas, distractibility, hyperactivity, or excessive involvement in risky activities).”[2] In mania, various pharmaceutical approaches have been researched. The first-line recommendation includes mood stabilizers (MS) like lithium and atypical antipsychotics like quetiapine but frequent side effects like weight gain, metabolic dysregulation, and sedation are reported which results in approximately 50% non-adherence.[3-7] The lifetime prevalence of bipolar disorder is between 0.1% and 4.4%.[8]
Electro-convulsive therapy (ECT) is used to treat mania and depression. Transcranial magnetic stimulation (TMS) being a non-invasive analog to electrical stimulation, we intended to use this quality to treat mania.[9] The lack of anesthesia requirements for the patient and the absence of the need to produce seizures are two benefits of repetitive TMS (rTMS) over ECT. rTMS, in contrast to ECT, has been found to enhance cognitive abilities, including both short- and long-term memory.[10]
In the current era, rTMS is a non-invasive alternative to electrical stimulation and is a viable therapeutic tool for refractory neuropsychiatric diseases based on neural network modulations.[11-13]
Theta burst stimulation (TBS) is a relatively new rTMS protocol that modulates activity in the underlying region in a shorter amount of time to enable more effective and long-lasting post-stimulation effects when compared with traditional rTMS.[14] McGirr et al. employed intermittent TBS (iTBS) therapy replacing the traditional protocol due to shortened delivery time targeting the left dorsolateral prefrontal cortex (LDLPFC) in acute bipolar depression.[15]
Literature with TMS remains scarce. In a controlled study by Grisaru et al., the results demonstrated that when employing fast rTMS, the right DLPFC is a superior target than the left DLPFC.[9] Praharaj et al. and colleagues in 2009 demonstrated a significant reduction in the Youngs Mania Rating Scale (YMRS) on undergoing daily stimulation of active or sham rTMS over the right dorsolateral prefrontal cortex for 10 days.[16] Kapstan and colleagues (2003), however, failed to distinguish between active rTMS and sham rTMS over the right DLPFC.[17]
The right prefrontal cortex’s cortical activity was found to be lower in mania in neuroimaging investigations.[18] The effect of available rTMS studies in Mania is inconclusive and TBS trials in acute phase mania have not been reported to date.[19] We intend to study the stronger, neuroplastic, robust, and short-duration protocol that is TBS with a large size and better study design stimulating bilateral sequential DLPFC.
MATERIAL AND METHODS
Subjects
To participate in the study, the right-handed patients between the ages of 18 and 59 were recruited. Individuals who met the diagnostic requirements for mania according to the International Classification of Diseases-tenth edition (ICD-10) (World Health Organization, 1992) were contacted.[20] The effect size calculated of prior randomized controlled trial (RCTs) by Kaptsan et al. (Cohen’s d = 0.29) and Praharaj et al. ([Cohen’s d = 0.74) is 0.51 (avg. Cohen’s d).[16,17] With the expectation of a similar modest effect size, number of groups, and repetition of evaluation being 2 and 3, using G-power and a critical F of 3.175, the total sample size was calculated to be 40 participants with an alpha error of 0.05, an anticipated 25% dropout rate and 1-beta error of 0.95. We enrolled 20 subjects in the “active” group and another 20 in the “sham” group [Figure 1]. Subjects with at least moderate-to-severe illness: Baseline YMRS Score >26 were included in the study.[21]

- Flowchart displaying the participant requirement process ‘ITT: Intension to treat analysis’
Exclusion criteria included any comorbid psychosis or substance use disorders excluding caffeine and nicotine (according to ICD-10 DCR), a history that suggests a brain injury or surgery, any substantial brain malformation or tumor, neurodegenerative disease or history of seizures, subjects who have received ECT in the preceding 6 months, and subjects with metallic parts in the body, such as pacemakers.
The research has been listed in the Clinical Trials Registry India (CTRI), Number: CTRI/2022/04/041957. The research was conducted at the psychiatry department, Sri Guru Ram Rai Institute of Medical and Health Sciences, Dehradun. The ethical committee of the institution approved the study protocol. (SGRR/IEC/2/19; IEC Registration No. ECR/710/ Inst/UK/2015/RR-18).
Seventy-two patients were screened for the study and among them, 40 patients in all were signed up. The patients were all admitted to the inpatients in the department of psychiatry and underwent regular general physical and systemic examination, basic metabolic profile, and electrocardiography. Block randomization was used to assign patients at random to the active and sham groups. The patients were hospitalized for 2 weeks during which iTBS and cTBS sessions were given. The follow-up evaluation was conducted post-discharge, on outpatient visits. A detailed written informed consent was signed by the patient and their caregivers before enrolment, after explaining the full procedure in detail along with the expected benefits and risks involved. The patients continued the psychotropics for the complete study duration. No psychotherapy was provided for any of the patients enlisted in the study.
Two patients in the active group and where as one in the sham group discontinued the study (1 – worsening of symptoms and 2 – uncooperative). Nobody among the patients discontinued treatment as a result of rTMS side effects.
rTMS stimulation parameters
TBS was done using a “MagVenture - MagPro- R30.” Theta Burst’s booster and the figure-of-8-shaped B65 coil are both found in the MagPro- R30 device. The dorsol lateral prefrontal cortex was the favored stimulation location. The worldwide 10–20 Electroencephalography (EEG) system was utilized for the positioning of the TMS coil [Figure 2]. The system was chosen since it often appears in other clinical TMS applications and takes participant skull size diversity into account.[22]

- Site of theta burst stimulation stimulation as per international 10–20 system
There were 20 TBS sessions in total for both groups (two sessions/day, first left TBS and after 30 min right TBS, a rigorous protocol for 5 days a week, intensive protocol in accordance with prior studies in other disorders [Figure 3].[23]
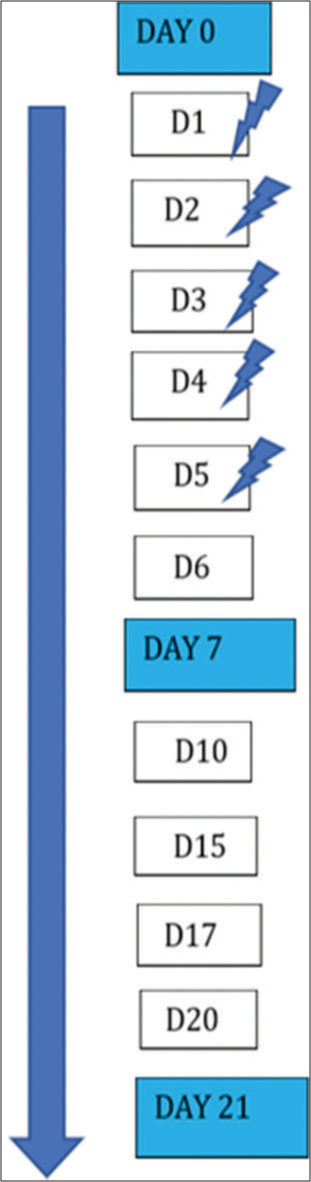
- Days of stimulation.
An active cTBS protocol burst of three pulses was used, delivered at 50 Hz, which was repeated every 200 MS (at a frequency of 5 Hz) lasting 40 s and containing a total of 600 pulses.
The iTBS, which delivered a total of 600 pulses in 20 trains of 10 bursts at intervals of 8 s and 3 pulses at 50 Hz, repeated at a rate of 5 Hz. A total of 1200 pulses were delivered/day. Instead of the standard 600 pulses/day, as suggested by Huang et al. and colleagues, we provided 1200 pulses/day.[24]
We delivered a stronger version of TBS of rTMS with a large size and better study design stimulating both right and left DLPFC.
We used TBS at 80% of the resting motor threshold (RMT) since the plasticity of a stimulus is affected by its intensity. Before each session of stimulation, this was established. with the lowest intensity according to the Rossini-Rothwell method. In each of the ten trials, RMT generated five MEP responses that were at least 50 uV.[25,26] Sham rTM was delivered using the opposite side of the coil of 8, which resembles active stimulation in terms of sound and scalp contact. The previous research has utilized a sham stimulation technique similar to this one.[27,28]
Clinical measures
The intensity of manic symptoms was evaluated by YMRS. The YMRS gives each item a severity rating. Irritability, thought content, speech, and disruptive/aggressive behavior are the four categories that are assessed on a 0–8 scale, and the remaining seven items are scored on a 0–4 scale.[29]
Clinical global impression (CGI)-Bipolar illness: For evaluating the severity of manic episodes the degree of change from the illness’s worst phase and the phase that came right before it.[30] A tailored checklist based on rTMS-related unfavorable events from prior studies was used to assess rTMS-related adverse events after each session.
Blinding procedure
An impartial evaluator who was unaware of the randomization of the stimulation groups into active or sham conditions evaluated the subjects at baseline, 7th day, and on day 21. Blocks with random-number patterns were used to divide the patients into active and sham groups. There were unopened envelopes with these numbers. The clinician who gave the TBS immediately before the first session for each patient opened the envelope. In addition, the patients were unaware of the treatment arm. The rater was required to retrospectively infer the treatment arm for each trial participant based on the pre- and post-psychopathological ratings. A Cohen’s Kappa coefficient of −0.016 for the guess matrix[31] indicated “no agreement,” which is a sign of the blind’s good integrity. Independent verification of blinding of participants to treatment arms was not possible.[27,28]
Statistical analysis
SPSS was used to analyze the study’s data (Version 28). YMRS score served as the main outcome variable. Scores on the CGIBP-S, CGIBP-P, and CGIBP-W were secondary outcome factors. The Kolmogorov–Smirnov test and normal probability plots were used to confirm the assumption of normality. Independent t-tests and Chi-square tests were used to look at group differences in sample characteristics (wherever applicable). Using the Mann–Whitney U test, variables such as the number of episodes and motor threshold that violated the assumption of normality were compared. Regardless of whether a patient completed the trial or not, all of the patients who were enlisted were added to the analysis using the intention-to-treat (ITT) method. The latest observation carried forward approach was used to replace the missing data. In the double-blind phase, the primary analysis focused on the interaction effect of treatment over time (baseline, 7th, and 21st days) between the active and sham group. Using the restricted maximum likelihood mixed (growth curve) model analysis, the overall treatment effect over time for the two groups was examined, with time (0, 7th, and 21st) as the within-subject component, treatment (active/sham) serving as the between-subject factor, with allocation order serving as the “subjects.”
RESULTS
Sample characteristics
Comparable characteristics between the two groups included gender, marital status, religion, habitat, employment, socioeconomic status, and education. Medical morbidity (χ2 (1, 40) = 2.125, P = 0.764) and number of episodes (U = 226.500, P = 0.478) present in comparable proportions in both the “active” and “sham” group individuals. The subjects randomly assigned to the “active” and “sham” groups were comparable in age (t (40) = 0.041, P = 0.778), Motor Threshold (t (40) = 0.229, P = 0.400), and antipsychotic dose per trial (As determined by Woods, the chlorpromazine equivalent dose) [Table 1].[32]
| Variables | Active (n=20) mean±SD/n (%) | Sham (n=20) mean±SD/n (%) | χ2/t/U | Df | P |
|---|---|---|---|---|---|
| Age (in years) | 32.75±11.60 | 32.90±11.81 | 0.041 | 38 | 0.778 |
| Number of episodes | 1.90±1.89 | 4.60±5.95 | 226.500# | -- | 0.478 |
| Motor threshold | 39.75±2.94 | 39.95±2.56 | 0.229 | 38 | 0.400 |
| Gender | |||||
| Male | 12 (30) | 14 (35) | 0.440 | 1 | 0.507 |
| Female | 8 (20) | 6 (15) | |||
| Marital status | |||||
| Married | 13 (32.5) | 9 (22.5) | 1.616 | 1 | 0.204 |
| Unmarried | 7 (17.5) | 11 (27.5) | |||
| Religion | |||||
| Hindu | 17 (42.5) | 17 (42.5) | 0.000& | 1 | 1.000 |
| Muslim | 3 (7.5) | 3 (7.5) | |||
| Habitat | |||||
| Urban | 14 (35) | 14 (35) | 0.000 | 1 | 1.000 |
| Rural | 6 (15) | 6 (15) | |||
| Socioeconomic status | |||||
| Upper | 6 (15) | 3 (7.5) | 1.448& | 2 | 0.589 |
| Middle | 10 (25) | 11 (27.5) | |||
| Lower | 4 (10) | 6 (15) | |||
| Occupation | |||||
| Employed | 9 (22.5) | 10 (25) | 0.100 | 1 | 0.752 |
| Unemployed | 11 (27.5) | 10 (25) | |||
| Education | |||||
| Illiterate | 5 (12.5) | 3 (7.5) | 1.817& | 4 | 0.815 |
| Primary | 5 (12.5) | 8 (20) | |||
| Secondary | 2 (5) | 3 (7.5) | |||
| Graduate | 6 (15) | 5 (12.5) | |||
| Postgraduate | 2 (5) | 1 (2.5) | |||
| Comorbidity | |||||
| Absent | 15 (37.5) | 17 (42.5) | 2.125& | 3 | 0.764 |
| Hypertension | 2 (5) | 2 (5) | |||
| Diabetes mellitus | 2 (5) | 0 (0) | |||
| Hypothyroid | 1 (2.5) | 1 (2.5) | |||
| Antipsychotic | |||||
| Olanzapine | 10 (25) | 10 (25) | |||
| Risperidone | 3 (7.5) | 4 (10) | |||
| Trifluoperazine | 1 (2.5) | 3 (7.5) | 2.429& | 4 | 0.702 |
| Clozapine | 5 (12.5) | 2 (5) | |||
| Quetiapine | 1 (2.5) | 1 (2.5) | |||
| Mood stabilizer | |||||
| Valproate | 13 (32.5) | 11 (27.5) | |||
| Lithium | 7 (17.5) | 5 (12.5) | 4.500& | 2 | 0.177 |
| Valproate+Lithium | 0 (0) | 4 (10) | |||
| Benzodiazepine used | |||||
| Yes | 12 (30) | 12 (30) | 0.000 | 1 | 1.000 |
| No | 8 (20) | 8 (20) | |||
| CPZ dose (last trial) | 266.25±82.03 | 285.00±60.91 | 247.500# | -- | 0.201 |
Safety and side effects
TBS was not associated with any severe adverse effects. Nine patients (six from the “active” group and three from the “sham”) complained of headaches in the early sessions, which were relieved by analgesics. There are no reports of any people quitting because of side effects.
Outcome measures
At the baseline psychopathology scores such as YMRS (t = 0.081; P = 3.280), CGIBP-S (t = 1.295; P = 0.136), CBIBP-P (t = 0.309; P = 0.192), and CGIBP-W (t = 0.312; P = 0.354) were comparable for both the “active” and “sham” groups.
[Table 2] compares the pre-post effects from the pre-treatment period, 7th and 21st across the two groups, taking into account the effects of group and time on the ITT analysis.
| Variables | A mean±SD | B mean±SD | C mean±SD | F@ | P | Partial eta2 |
|---|---|---|---|---|---|---|
| YMRS | ||||||
| Active | 39.45±3.35 | 27.65±6.48 | 18.60±9.13 | 0.300 | 0.612 | 0.008 |
| Sham | 39.55±4.39 | 29.00±5.77 | 19.15±6.95 | |||
| CGIBP-S | ||||||
| Active | 4.80±0.83 | 3.40±0.75 | 2.30±1.129 | 0.432 | 0.562 | 0.011 |
| Sham | 5.15±0.88 | 3.55±0.76 | 2.45±0.95 | |||
| CGIBP-P | ||||||
| Active | 3.45±0.51 | 2.55±0.69 | 1.90±0.85 | 0.202 | 0.701 | 0.005 |
| Sham | 3.50±0.51 | 2.55±0.61 | 2.20±0.56 | |||
| CGIBP-W | ||||||
| Active | 3.40±0.50 | 2.50±0.69 | 1.90±0.85 | 0.200 | 0.705 | 0.005 |
| Sham | 3.45±0.51 | 2.60±0.60 | 2.00±0.56 |
A: 0 day, B: 7th day, C: 21st day, P<0.05 level (2 tailed). YMRS: Youngs mania rating scale, CGIBP-S: Clinical global impression-bipolar disorder-severity of illness, CGIBP-P: Clinical global impression-bipolar disorder-change from preceding phase, CGIBP: W: Clinical global impression-bipolar disorder change from worst phase of illness
Repeated measures-ANOVA did not find a significant group*time effect for each of the variables, that is, YMRS (F = 0.300; P ≤ 0.612), CGIBP-S (F = 0.432; P = 0.562), CGIBP-P (F = 0.202; P = 0.701), and CGIBP-W (F = 200; P =0.705).
According to YMRS scores, 85% of the active group and 80% of the sham group responded. The active group had a 1.417 odds ratio for response when compared to the sham group.
A drop in the overall YMRS scores of at least 50% from baseline till day 21 was taken into account as a response.[33]
DISCUSSION
The current research is a RCT that targets the right and left prefrontal cortex with iTBS and cTBS for treating acute mania.
According to Michael and Erfurth, high-frequency rTMS monotherapy delivered to the right DLPFC may be useful in treating manic bipolar patients (2004).[34]
In patients with mania, it was believed that there is a relative increase in metabolism on the left side and whereas the right side has a lower anterior metabolism (hypofrontality), unlike what is observed with depression.[35] The mechanisms of TMS’s actions are not well understood. However, a key theory in the field of TMS has been that slow rTMS has an inhibiting effect on physiological changes, whereas fast rTMS causes excitatory changes effect. TMS may be equally helpful in treating depression in people by either by activating their left hemisphere or by suppressing their right hemisphere. In mania, the reverse would take place, indicating that high-frequency rTMS would have a greater therapeutic effect when the right DLPFC was activated.[36,37] Deckersbach et al. demonstrated increased rCBF in the LDLPFC among bipolar patients.[38] In our study, we aimed to correct the altered blood flow or metabolism.
Our study utilized cTBS (inhibitory) over left DLPFC and iTBS (stimulatory) over right DLPFC, at 50 Hz twice daily for 5 days in 2 consecutive weeks. We stimulated the right prefrontal cortex as in prior studies but used an inhibitory protocol for the left prefrontal cortex considering that left stimulation could worsen manic symptoms. We gave a total of 20 sessions for each patient.
The patients were assessed for baseline YMRS, CGIBP-S, CBIBP- P, and CGIBP-W before stimulation or placebo stimulation. The baseline measurements were statistically different in both groups. To reduce the total treatment time, an intensive TBS procedure was used. Other psychiatric disorders have been successfully studied using similar intensive and accelerated protocols.[27,28] Despite employing a significantly higher stimulation regimen, TBS was well tolerated and only briefly caused minor side effects like a headache. No participant in the research reported quitting the experiment due to side effects. While unable to draw the conclusion that adjunctive TBS is more effective than a placebo in treating mania, this study illustrates the tolerability and safety of TBS activating PFC in mania.
We suggest potential flaws in our research that may have contributed to this result. First, we stimulated the vermis at a lower intensity (80% RMT), as suggested by Huang et al.[24]
Second, we delivered 1200 pulses/day. But a higher stimulation dose of 2400 pulses/day have been delivered in other major psychiatric disorders.[39] We failed to assess the clinical subtype of the manic episode (dysphoric/mixed vs. euphoric) and the underlying temperament.
Third, state-based variables were not kept homogenous. Further research is needed to maximize the effects of stimulation by exploiting the variability in the neuronal response to TMS. The efficient application of TMS can be improved by the direct monitoring of brain activity utilizing non-invasive techniques, such as EEG or hemodynamic-based imaging.[40]
A scalp-based localization method can be replaced with neuronavigation to target a particular cortical location. In addition, as proposed in other mental illnesses like OCD, TMS treatment can be tailored using biophysical models to change the ideal angle of stimulation.[41]
CONCLUSION
According to the results of our trial, intensive TBS is safe and well tolerated but does not significantly alter mania psychopathology as compared to the cases receiving a placebo or sham treatment. It may be needed to conduct studies utilizing the neuronavigation-based localization technique with a large sample, a longer time frame, and improved dosage regimens.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Financial support and sponsorship
Nil.
References
- Aripiprazole alone or in combination for acute mania. Cochrane Database Syst Rev. 2013;12:CD005000.
- [CrossRef] [Google Scholar]
- Diagnostic and Statistical Manual of Mental Disorders. (5th ed). Arlington: American Psychiatric Association; 2013.
- [CrossRef] [Google Scholar]
- The international college of neuropsychopharmacology (CINP) treatment guidelines for bipolar disorder in adults (CINP-BD-2017), Part 3: The clinical guidelines. Int J Neuropsychopharmacol. 2017;20:180-95.
- [CrossRef] [PubMed] [Google Scholar]
- Managing the side effects associated with commonly used treatments for bipolar depression. J Affect Disord. 2014;169(Suppl 1):S34-44.
- [CrossRef] [PubMed] [Google Scholar]
- Outcome in the pharmacologic treatment of bipolar disorder. J Clin Psychopharmacol. 1996;16:15S-23.
- [CrossRef] [PubMed] [Google Scholar]
- Self-reported adherence to treatment with mood stabilizers, plasma levels, and psychiatric hospitalization. Am J Psychiatry. 2002;159:1927-9.
- [CrossRef] [PubMed] [Google Scholar]
- Bipolar affective disorder: Pharmacotherapeutic profile and adherence to medication. Rev Esc Enferm USP. 2012;46:689-95.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence and correlates of bipolar spectrum disorder in the world mental health survey initiative. Arch Gen Psychiatry. 2011;68:241-51.
- [CrossRef] [PubMed] [Google Scholar]
- Transcranial magnetic stimulation in mania: A controlled study. Am J Psychiatry. 1998;155:1608-10.
- [CrossRef] [PubMed] [Google Scholar]
- Comparing the effects of repetitive transcranial magnetic stimulation and electroconvulsive therapy in the treatment of depression: A systematic review and meta-analysis. Depress Res Treat. 2014;2014:135049.
- [CrossRef] [PubMed] [Google Scholar]
- Rapid-rate transcranial magnetic stimulation of left dorsolateral prefrontal cortex in drug-resistant depression. Lancet. 1996;348:233-7.
- [CrossRef] [PubMed] [Google Scholar]
- A controlled trial of daily left prefrontal cortex TMS for treating depression. Biol Psychiatry. 2000;48:962-70.
- [CrossRef] [PubMed] [Google Scholar]
- Transcranial magnetic stimulation in neurology. Lancet Neurol. 2003;2:145-56.
- [CrossRef] [PubMed] [Google Scholar]
- Theta-burst stimulation: Remote physiological and local behavioral aftereffects. Neuroimage. 2008;40:265-74.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy of active vs sham intermittent theta burst transcranial magnetic stimulation for patients with bipolar depression: A randomized clinical trial. JAMA Netw Open. 2021;4:e210963.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy of high frequency (rapid) suprathreshold repetitive transcranial magnetic stimulation of right prefrontal cortex in bipolar mania: A randomized sham controlled study. J Affect Disord. 2009;117:146-50.
- [CrossRef] [PubMed] [Google Scholar]
- Right prefrontal TMS versus sham treatment of mania: A controlled study. Bipolar Disord. 2003;5:36-9.
- [CrossRef] [PubMed] [Google Scholar]
- Decision-making in mania: A PET study. Brain. 2001;124:2550-63.
- [CrossRef] [PubMed] [Google Scholar]
- A systematic review and meta-analysis of randomized sham-controlled trials of repetitive transcranial magnetic stimulation for bipolar disorder. Psychiatr Q. 2020;91:1225-47.
- [CrossRef] [PubMed] [Google Scholar]
- The ICD-10 Classification of Mental and Behavioral Disorders: Clinical Descriptions and Diagnostic Guidelines Geneva: World Health Organization; 2006. p. :159.
- [Google Scholar]
- Relationship between the clinical global impression of severity for schizoaffective disorder scale and established mood scales for mania and depression. J Affect Disord. 2013;150:17-22.
- [CrossRef] [PubMed] [Google Scholar]
- Left frontal pole theta burst stimulation decreases orbitofrontal and insula activity in cocaine users and alcohol users. Drug Alcohol Depend. 2017;178:310-7.
- [CrossRef] [PubMed] [Google Scholar]
- Safety and efficacy of continuous theta burst “intensive” stimulation in acute-phase bipolar depression: A pilot, exploratory study. J ECT. 2023;39:28-33.
- [CrossRef] [PubMed] [Google Scholar]
- Theta burst stimulation of the human motor cortex. Neuron. 2005;45:201-6.
- [CrossRef] [PubMed] [Google Scholar]
- Magnetic stimulation: Motor evoked potentials. The International Federation of Clinical Neurophysiology. Electroencephalogr Clin Neurophysiol Suppl. 1999;52:97-103.
- [Google Scholar]
- The efficacy of cerebellar vermal deep high frequency (theta range) repetitive transcranial magnetic stimulation (rTMS) in schizophrenia: A randomized rater blind-sham controlled study. Psychiatry Res. 2016;243:413-20.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy of intensive cerebellar intermittent theta burst stimulation (iCiTBS) in treatment-resistant schizophrenia: A randomized placebo-controlled study. Cerebellum. 2021;20:116-23.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy of intensive orbitofrontal continuous Theta Burst Stimulation (iOFcTBS) in Obsessive Compulsive Disorder: A Randomized Placebo Controlled Study. Psychiatry Res. 2021;298:113784.
- [CrossRef] [PubMed] [Google Scholar]
- Young mania rating scale In: Handbook of Psychiatric Measures. Washington, DC: American Psychiatric Association; 2000. p. :540-2.
- [Google Scholar]
- Modification of the Clinical Global Impressions (CGI) Scale for use in bipolar illness (BP): The CGI-BP. Psychiatry Res. 1997;73:159-71.
- [CrossRef] [PubMed] [Google Scholar]
- Assessing Blinding in Randomized Clinical Trials. 2017. Pomana: California State Polytechnic University; Available from: http://dspace.calstate.edu/bitstream/handle/10211.3/196699/WaiteJesse_Thesis2017.pdf?sequence=6 [Last assessed on 2022 Nov 15]
- [Google Scholar]
- Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2003;64:663-7.
- [CrossRef] [PubMed] [Google Scholar]
- Response to placebo among bipolar I disorder patients experiencing their first manic episode. Bipolar Disord. 2000;2:332-5.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment of bipolar mania with right prefrontal rapid transcranial magnetic stimulation. J Affect Disord. 2004;78:253-7.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy of adjunctive high frequency repetitive transcranial magnetic stimulation of right prefrontal cortex in adolescent mania: A randomized sham-controlled study. Clin Psychopharmacol Neurosci. 2015;13:245-9.
- [CrossRef] [PubMed] [Google Scholar]
- Functional cerebral asymmetry in affective disorders: New facts contributed by transcranial magnetic stimulation. J Affect Disord. 2001;66:103-9.
- [CrossRef] [PubMed] [Google Scholar]
- Interhemispheric asymmetry of motor cortical excitability in major depression as measured by transcranial magnetic stimulation. Br J Psychiatry. 2000;177:169-73.
- [CrossRef] [PubMed] [Google Scholar]
- Impaired recruitment of the dorsolateral prefrontal cortex and hippocampus during encoding in bipolar disorder. Biol Psychiatry. 2006;59:138-46.
- [CrossRef] [PubMed] [Google Scholar]
- Repetitive transcranial magnetic stimulation for psychiatric symptoms in long-term hospitalized veterans with schizophrenia: A randomized double-blind controlled trial. Front Psychiatry. 2022;13:873057.
- [CrossRef] [PubMed] [Google Scholar]
- State-dependent variability of neuronal responses to transcranial magnetic stimulation of the visual cortex. Neuron. 2009;62:291-303.
- [CrossRef] [PubMed] [Google Scholar]
- Transcranial magnetic stimulation in obsessive-compulsive disorder: A focus on network mechanisms and state dependence. Neuroimage Clin. 2018;19:661-74.
- [CrossRef] [PubMed] [Google Scholar]








