Translate this page into:
Rash decisions: A case report of Risperidone and exanthematous eruptions

*Corresponding author: Sukriti Mukherjee, Department of Psychiatry, All India Institute of Medical Sciences, Kalyani, West Bengal, India. mukherjeesukriti@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Maity M, Mukherjee S. Rash decisions: A case report of risperidone and exanthematous eruptions. Arch Biol Psychiatry. 2024;2:45-7. doi: 10.25259/ABP_41_2023
Abstract
Here, we describe a clinical case involving a 19-year-old male diagnosed with schizophrenia and initiated on risperidone 2 mg/day. The dose was gradually built up to 6 mg/day. Within a week, the patient manifested an extensive exanthematous eruption characterized by collarette-shaped desquamation and intense pruritus. The medication was promptly discontinued, and aripiprazole was introduced, resulting in the complete resolution of the cutaneous manifestations within the subsequent week.
Keywords
Antipsychotics
Risperidone
Exanthematous eruptions
Schizophrenia
Adverse drug reaction
INTRODUCTION
Antipsychotic agents commonly trigger cutaneous reactions in approximately 5% of users, often presenting as exanthematous eruptions, skin pigmentation alterations, photosensitivity, urticaria, and pruritus.[1] Risperidone is an atypical antipsychotic primarily targeting 5-HT2 and D2 receptors, and risperidone-associated cutaneous reactions are infrequent, but isolated instances of cutaneous adverse reactions have been documented.[2]
CASE REPORT
A 19-year-old male, presented to the outpatient department with persecutory delusion against his neighbors and second-person commenting-type auditory hallucination for 8 months, along with impaired biological and socio-occupational functioning for a similar duration. A diagnosis of schizophrenia was assigned, and he was started on risperidone 2 mg/day and was hiked up to 4 mg/day with a plan of up-titration to 6 mg/day if needed on subsequent visits. The patient came back after 7 days with extensive exanthematous lesions all over the body, including arms [Figure 1a], abdominal region [Figure 1b], and back [Figure 1c], which started after 2 days of starting risperidone. The eruptions started as an acute rash with pinhead-sized pustules with an erythematous base. The rash started in the elbow region and extended to the trunk and the whole upper limb within a span of 48 hours. The rash was associated with severe itching and a burning sensation. Mucosal involvement was not present. On examination, collarette-shaped desquamation was observed. A dermatological opinion was taken that concurred with the advice from our side and did not add any medication. Risperidone was stopped, and the patient was started on aripiprazole 10 mg/day and was advised for a follow-up after 7 days. On the next visit, there were no lesions found in the patient.
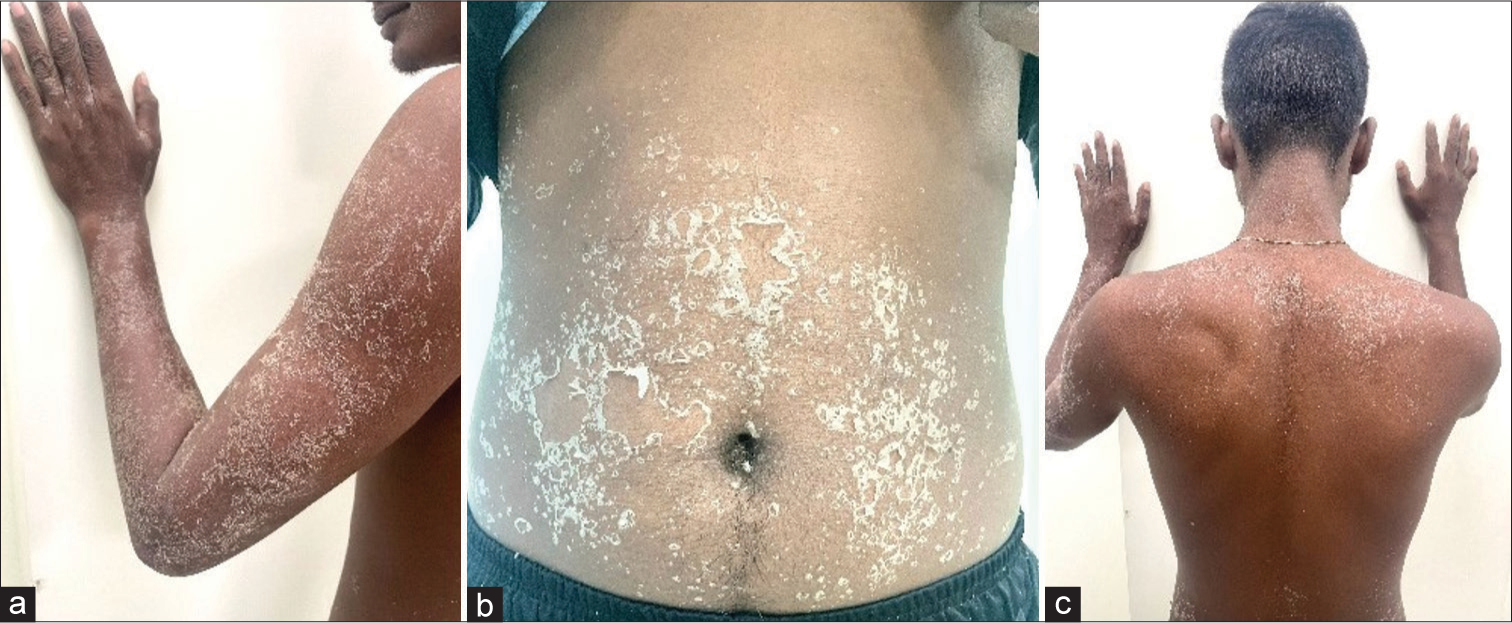
- Exanthematous eruptions over different areas of the body, (a): Lesions over the arms, (b): Lesions over the abdominal region, (c): Lesions over back.
A score of 6 on the Naranjo causality assessment scale was found after evaluation, which indicates that the drug is the possible agent for the skin eruption [Figure 1].
DISCUSSION
A recent literature search highlighted a singular case from Korea where a 4 mg/day oral risperidone solution led to a facial-specific acute cutaneous syndrome.[3] An Indian case involving urticaria on the face and neck, characterized by erythematous patches with raised ridges with risperidone 3 mg/day, was reported earlier.[4] Another case reported incidents of rashes when risperidone was started at a dosage of 6 mg/day. The rashes did not resolve with a lower dosage, and ultimately, the lesions resolved after the medicine was stopped.[5]
The most prevalent form of drug eruptions is characterized as maculopapular rash, exhibiting erythematous macules that evolve into papules ranging from 1 to 5 mm in diameter, potentially merging into plaques.[6] Notably, the timing of lesion occurrence is distinct, with the initial appearance following a sensitization phase, usually 5–14 days after treatment initiation and occasionally after drug cessation.[7] Maculopapular rash typically affects the face, neck, or upper trunk, spreading symmetrically toward the limbs. In general, it is self-limiting and subsides within 7–14 days upon discontinuing the causal medication.[6]
Several theories might explain our case of delayed hypersensitivity reaction. The hapten hypothesis postulates haptens forming hapten-protein complexes and inducing immunoglobulin (Ig) E or IgG-mediated responses. The pharmacological hypothesis mentions that interaction occurs through reversible drug binding to human leukocyte antigens or T-cell receptors, bypassing antigen-processing pathways. The pseudo-allergic hypothesis mentions that responses are not immune-mediated but linked to a drug’s pharmacological or toxicological properties and genetic predisposition. Although there are several theories, there is an absence of a strong evidence base to postulate the exact mechanism behind adverse reactions in our patients.[8,9]
CONCLUSION
Clinicians should remain vigilant about the rare complication of skin eruptions with risperidone at a relatively higher dose, especially during the first few weeks. Although no serious complications such as toxic epidermal necrolysis or Stevens-Johnson syndrome have been reported with risperidone to date, eruptions can be a reason for non-adherence to the treatment.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that they have used artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript or image creations.
Financial support and sponsorship
Nil.
References
- Adverse cutaneous reactions to antipsychotics. Am J Clin Dermatol. 2002;3:629-36.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy and safety of risperidone long-acting injection in elderly people with schizophrenia. Clin Interv Aging. 2009;4:351-5.
- [CrossRef] [PubMed] [Google Scholar]
- Rash and desquamation associated with risperidone oral solution. Prim Care Companion J Clin Psychiatry. 2008;10:414-5.
- [CrossRef] [PubMed] [Google Scholar]
- Risperidone-induced recurrent giant urticaria. Br J Clin Pharmacol. 2007;64:558-9.
- [CrossRef] [PubMed] [Google Scholar]
- Risperidone-induced skin rash. Indian J Psychiatry. 2016;58:106.
- [CrossRef] [PubMed] [Google Scholar]
- Drug eruption reference manual: With CD-ROM (9th ed). Boca Raton, FL: Parthenon; 2003.
- [Google Scholar]
- Recent developments and highlights in drug hypersensitivity. Allergy. 2019;74:2368-81.
- [CrossRef] [PubMed] [Google Scholar]
- Mechanisms of hypersensitivity reactions induced by drugs. Acta Biomed. 2019;90:44-51.
- [Google Scholar]








