Translate this page into:
Emergence of nail hyperpigmentation possibly linked to fluoxetine: A case report

*Corresponding author: Lalit Vijay Hirwe, Department of Psychiatry, Dr. Vasantrao Pawar Medical College, Hospital and Research Centre, Nashik, Maharashtra, India. lalithirwe.psydr@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Hirwe LV, Bharati AS. Emergence of nail hyperpigmentation possibly linked to fluoxetine: A case report. Arch Biol Psychiatry. 2024;2:78-80. doi: 10.25259/ABP_8_2024
Abstract
This case report describes a rare presentation of nail pigmentation in a 42-year-old male patient with a history of depression. The patient was prescribed fluoxetine for his depressive symptoms and clonazepam for sleep disturbances. After 1 month of fluoxetine treatment, the patient developed nail pigmentation on both hands. Dermatological evaluation ruled out underlying diseases or allergies but suggested a potential drug reaction. Fluoxetine was discontinued, and an alternative antidepressant was initiated. Remarkably, the nail pigmentation resolved within 1–2 weeks of discontinuing fluoxetine. This case highlights the importance of recognizing and managing uncommon medication-related reactions to ensure patient well-being.
Keywords
Case report
Fluoxetine
Hyperpigmentation
Selective serotonin reuptake inhibitors
Stereoisomerism
INTRODUCTION
Nail abnormalities are known to occur in various medical conditions, including dermatological disorders, systemic diseases, and medication reactions. While antidepressants, particularly selective serotonin reuptake inhibitors (SSRIs), are widely used in the management of depression, unusual cutaneous reactions are reported infrequently. We present a case of nail pigmentation that emerged during fluoxetine treatment.
CASE REPORT
A 42-year-old male presented with a history of depression, persisting for 7–8 months. He was initiated on fluoxetine 20 mg in the morning for depressive symptoms and clonazepam 0.5 mg for sleep disturbances. Gradually, the dose of clonazepam was decreased and stopped as there were no insomnia complaints. The dose of fluoxetine was increased to 40 mg OD according to symptoms. After a month of therapy, the patient reported the emergence of nail pigmentation affecting both upper limbs [Figure 1]. The pigmentation was not accompanied by pain, itching, or any other related symptoms. Concerned about the change in his nail color, the patient sought dermatological consultation.
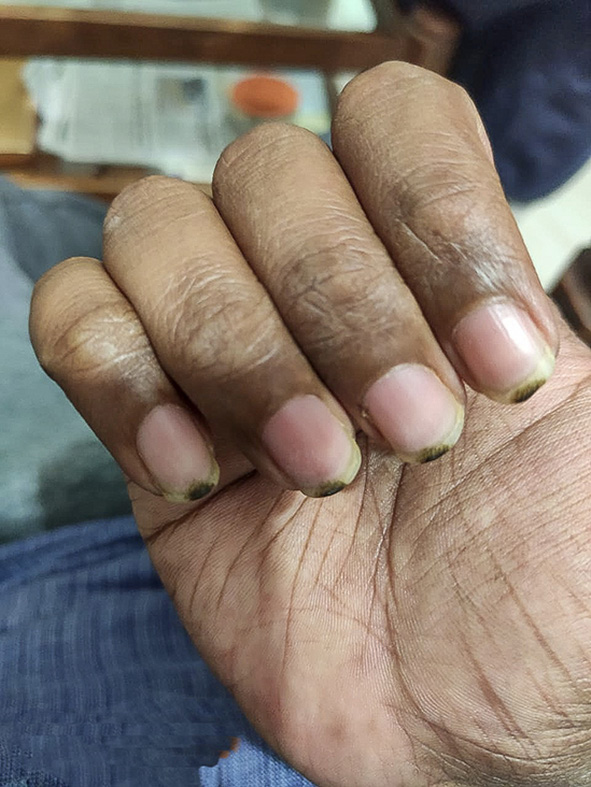
- Nail hyperpigmentation caused in a patient due to fluoxetine use.
Investigations
The dermatological assessment revealed blackish-pigmented spots over nails on both upper limbs. The dermatologist conducted a comprehensive evaluation, including a detailed medical history and a thorough physical examination. No evidence of underlying dermatological diseases or allergies was observed. The dermatologist postulated a potential drug reaction as the cause of the nail pigmentation, with fluoxetine being the prime suspect as the patient was not on any other drugs in that period. To confirm the hypothesis, we applied the Naranjo Adverse Reaction Probability Scale, where we found a score of 6, suggesting probable adverse drug reactions due to fluoxetine.[1]
Management
Fluoxetine was discontinued. Over 1–2 weeks following the discontinuation of fluoxetine, the patient reported gradual fading of the nail pigmentation [Figure 2], which eventually returned to normal. The patient was then transitioned to an alternative antidepressant regimen. We started on Desvenlafaxine 50 mg at night. The resolution of hyperpigmentation was correlated with the timeline of fluoxetine cessation, reinforcing the possibility of a drug-induced reaction.

- Nail pigmentation reduced after stopping the fluoxetine use.
DISCUSSION
The emergence of nail pigmentation, in this case following fluoxetine administration, suggests a potential adverse drug reaction. It has been reported that antidepressant drug reactions result in adverse skin reactions such as hyperpigmentation of the skin.[2] Psychotropic medications have been found to cause 0.1% of drug-induced cutaneous responses. Several kinds of psychotropic medications are involved in clinically significant cutaneous drug responses; mood stabilizers account for 39% of these reactions, antidepressants for 29%, and neuroleptics for 19%.[3] In a study tracking 109,000 individuals, the incidence of cutaneous drug responses with antidepressant therapy was 0.054%.[3] In the literature review, we could not find any such case reporting specific nail pigmentation caused by fluoxetine like this. Fluoxetine is linked to melanin synthesis.[4] The fluoxetine can up-regulate melanin synthesis in B16F10 melanoma cells and normal human melanocytes (NHM). Racemic fluoxetine consists of two stereoisomers. The R-fluoxetine increases melanin synthesis through a 5-HT1a/2a receptor and p38 mitogen-activated protein kinases (MAPKs) signaling pathways. Moreover, it is suggested that R-fluoxetine may be used as a drug for skin hypopigmentation disorders. [5] There are reports of various antidepressant drugs causing adverse cutaneous reactions, such as escitalopram, fluoxetine, sertraline, paroxetine, tricyclic antidepressants, venlafaxine, bupropion, and mirtazapine.[6] Furthermore, SSRIs are found to cause worsening of cutaneous lesions when switched from another SSRI, probably due to cross-sensitivity or cross-reactivity between the molecules.[7]
CONCLUSION
This case highlights the importance of recognizing unusual and non-life-threatening side effects or reactions associated with antidepressant medications. Clinicians should be attentive to patients’ reports of changes in physical appearance, such as skin and nail pigmentation, even if they are uncommon. While fluoxetine-induced nail pigmentation is a rare occurrence, it should be considered in the differential diagnosis of unexplained nail changes in patients receiving this medication. Further research is needed to elucidate the mechanisms underlying such reactions and to develop appropriate management strategies.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship: Nil.
References
- A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239-45.
- [CrossRef] [PubMed] [Google Scholar]
- Adverse cutaneous reactions to antidepressants. Am J Clin Dermatol. 2002;3:329-39.
- [CrossRef] [PubMed] [Google Scholar]
- Cutaneous adverse reactions to psychotropic drugs: Data from a multicenter surveillance program. J Clin Psychiatry. 2009;70:1258-65.
- [CrossRef] [PubMed] [Google Scholar]
- Up-regulation of melanin synthesis by the antidepressant fluoxetine. Exp Dermatol. 2012;21:635-7.
- [CrossRef] [PubMed] [Google Scholar]
- R-fluoxetine increases melanin synthesis through a 5-HT1A/2A receptor and p38 MAPK signaling pathways. Int J Mol Sci. 2018;20:80.
- [CrossRef] [PubMed] [Google Scholar]
- Dermatologic side effects of psychotropic medications. Psychosomatics. 2014;55:1-20.
- [CrossRef] [PubMed] [Google Scholar]
- Photosensitivity reaction associated with selective serotonin reuptake inhibitors: Possible cross-sensitivity? Psychiatry Clin Neurosci. 2014;68:244.
- [CrossRef] [PubMed] [Google Scholar]








